Breathing Room: Unlocking the Potential of Symbiosis for Coral Restoration
If you have been staring at a computer for the better part of the day, I’ll do you a favour now and paint a far nicer picture. So, channel your inner Jacques Cousteau, don your oval single lens SCUBA mask, and let’s dive headfirst into the world heritage listed, Great Barrier Reef. We are lucky today, not a breath of wind and the crystal-clear turquoise waters are a comfortable 26 degrees. Underwater, as your eyes adjust to the big blue, you notice a large shimmering blur ahead of you. Worryingly, this blur is pulsating, and far from static, it is making a rapid B-line towards you! Now near enough to touch, you see it is not one organism, but a school of inquisitive giant trevally. You’ve been spotted on the reef, a four-limbed neoprene alien. The call goes out and soon you are engulfed by like-minded schools of fish, contracting and swelling around you, friend or foe? Dive deeper, the fish lose interest and swim away. Finally, you see them. Corals. The unsuspecting life force of the entire reef. All around you, pinks, browns, greens, blues, boulders, brains, trees, fans. Halfway between slimy rocks and sea-anemones, these alien-like creatures are to reef ecosystems what trees are to our ancient rainforests. Among the 33 phyla existing on Earth, all but one are represented in these vibrant underwater cities, and 15 are found nowhere else. Welcome to the beating heart of the ocean.
You may be surprised to learn that corals have not always been the reef-building powerhouses we recognise today. Indeed, if we were to journey back 247 million years to our ancient oceans, we’d uncover a time where corals eked out a modest existence as humble reef dwellers, their role in reef-building overshadowed by the industrious efforts of sponges, calcified algae, and bryozoans. But corals are patient animals. And so they waited.
Fast forward a few million years, and we find ourselves in the Late Triassic era – a time of profound change. The once cool climate is beginning to warm, and as sea levels surge, coastlines begin to vanish beneath the waves giving rise to expansive underwater platform complexes. Amidst this shifting landscape the stage seemed set for corals to emerge from the shadows and ascend to their rightful place as architects of the reef. Almost. As it turns out, the road to reef-building mastery cannot be travelled alone. Corals needed a helping hand, a partner, a symbiotic alliance – one that would forever alter the course of their destiny.
And so it came to pass, on an otherwise ordinary day in our Mesozoic Ocean, when an ancient single-celled, photosynthetic micro-alga (zooxanthellae for ease) casually floating along, crossed paths with a humble coral. The coral, perhaps thinking it had found a snack, promptly ingested this zooxanthella. However, instead of being slowly digested, as is typical for most things that enter a coral’s mouth, this zooxanthella was, for reasons unknown, integrated into the coral’s gastrodermal cells, tucked safely away inside a specialised compartment now referred to as a symbiosome.

Fig. 1
Image of symbionts entering the cells of a coral
In the treacherous open waters of an ancient reef, the symbiosome must have been a safe haven to this zooxanthella; a place with a roof over its head and plenty of sunlight to boot. Add in some carbon dioxide, phosphates, and nitrogen compounds – the waste products from coral metabolism – and, more than a safe haven, you now have a photosynthetic organism’s paradise, a sunbed with a buffet. Safe and energized, this slightly confused but grateful zooxanthellae then proceeded to do what it does best: take energy from the sun and, through a series of complex photochemical reactions, produce a symphony of sugars, amino acids, and glycerol, which scientists collectively term, photosynthate.
In nature however, there is no such thing as a free lunch. In order to stay in paradise, the bulk of this hard earned photosynthate had to be dutifully transferred to the coral host in order to fuel its growth, immunity, and reproduction. Overall, this exchange must have been pleasing for both parties as it went on to lay the groundwork for a symbiotic partnership that would not only endure for millions of years but thrive.

Fig. 2
Image of zooxanthellae in a coral converting light to energy
Today, we witness the fruits of this ancient union: a staggering 778 coral-zooxanthellate dependent species spanning ~ 0.1% of Earth’s upper oceans. Ecosystem architects, corals and coral reefs now shape the livelihoods of millions of people, influence weather patterns, carbon cycling processes, and even impact the stability of global economies. These are the reef-builders which we know so well.
Thanks in part to their remarkable specialisation to a highly specific range of environmental conditions including temperature, pH, sunlight, and salinity, corals have maintained their dominance within the nutrient-poor waters of reef ecosystems for hundreds of millions of years. However, with extreme precision comes risk. For instance, even minor deviations in sea surface temperatures (SSTs), as slight as a 1.5°C increase, or prolonged exposure to brighter sunlight, can be enough to significantly impair the light-capturing ability of zooxanthellae. Much like taking a hammer to the solar panels on your neighbour’s roof, these zooxanthellae are left unable to “pay rent” and begin enjoying all the benefits of symbiosis while providing no return on investment.
In response to this, the coral swiftly seeks to sever ties. Within hours of symbiotic breakdown, coral polyps can be witnessed doing something remarkable, purging themselves of their once precious, now parasitic, zooxanthellae. Their sentence? To drift across the open ocean in search of a new home. Expelled.
Rid of their defunct brown solar panels, the coral begins to undergo a remarkable transition. Their once vibrant hues fade, their polyps retract, and in a matter of days, what is left resembles a haunting visage of literal skin and bones – the coral’s white skeleton visible through its translucent tissue. This is a bleached coral. Although still alive, the clock is most certainly ticking. If a bleached coral does not rapidly acquire nutrients – either through capturing and digesting food from the water column or by acquiring zooxanthellae better suited to the new conditions – it faces the grim prospect of a slow starvation and eventual death.
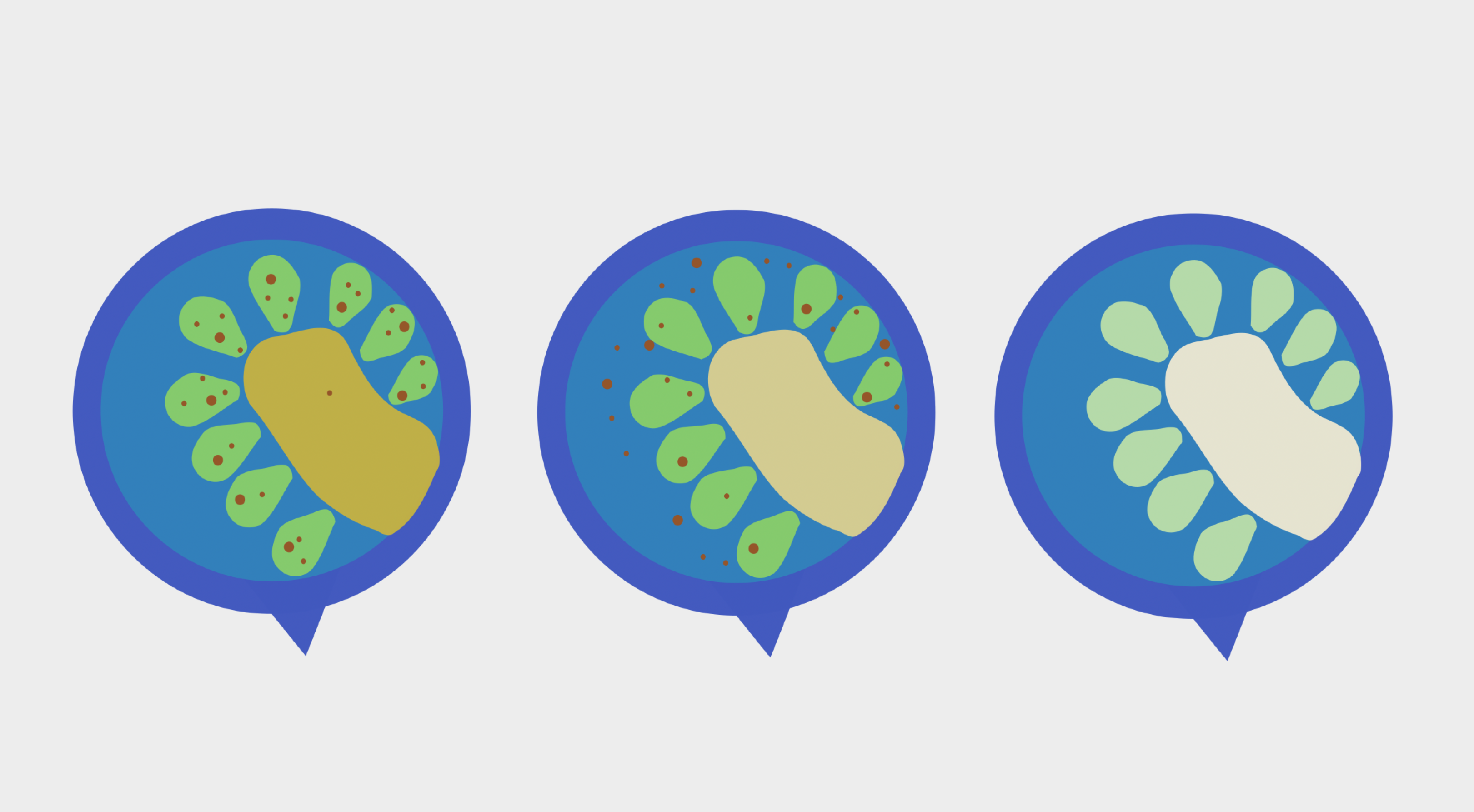
Fig. 3
Image showing the process of coral bleaching

Fig. 4
Image showing the process of coral bleaching
While a bleached coral is not necessarily cause for alarm, the situation grows markedly more perilous, when bleaching unfolds on an entire reef scale. This was most vividly illustrated during the Summer of 1998 – the first documented case of mass bleaching on the Great Barrier Reef and the first time ever coral bleaching was witnessed on a global scale. Now, some climate change sceptics often point out that bleaching events, like the one in 1998, have undoubtedly occurred countless times in Earth’s history without major loss. Indeed, they are not wrong. Historically, swift returns to favourable environmental conditions and adequate time between bleaching episodes have allowed bleached reefs to repopulate with zooxanthellae and – although a bit shaken up from the experience – make a full recovery. So why, then, have coral bleaching events escalated into a global catastrophe rather than a fascinating lesson in symbiotic breakdown?
Since the industrial revolution, our climate has undergone a formidable warming, 1.1°C to be exact. Without doubt, this alarming ascent can be directly linked to the unprecedented amounts of greenhouse gases entering Earth’s atmosphere – equivalent to the heat unleashed by approximately 600,000 daily detonations of Hiroshima bombs. Fortunately for us, the ocean has borne the brunt of this atmospheric onslaught, acting as a giant temperature buffer. However, now our vast blue sponge is starting to feel the heat with SSTs already rising by 0.74°C and ominous projections indicating a minimum 2°C increase by the end of the century. For coral reefs, the writing is on the wall: an unyielding surge in the severity, duration, and frequency of global marine heatwaves, and subsequently, mass bleaching events.
So while corals have indeed endured numerous marine warming events throughout Earth’s history, the pace of these events resembled more closely that of an ultra-marathon. Today, a 100-meter dash would be more accurate. And running in this race? We have in lane 5, the abstract concept of coral holobiont adaptive evolution, or the complex interplay of population genetics, natural selection, and underlying networks of biochemical constraints. Put simply: corals must adapt quickly or die. If you were a betting person, the odds may be too close for comfort. However, one contender is proving itself to be a dark horse, the corals’ partner in crime and our favourite photosynthetic microalgae: zooxanthellae.
Once upon a time, to mention zooxanthellae would be to refer solely to Symbiodinium microadriaticum Freudenthal, a monotypic genus with a world-wide distribution. However, during the early 1980’s it became clear that this species was comprised of a highly diverse group of organisms. Fast forwarding to today, we now recognise 15 genus-equivalent lineages distinguished by their widely divergent phylogenetic and physiological characteristics, and perhaps most importantly, by their variability in thermal tolerance.
As scientists began to delve into the surprising diversity of thermal tolerances among these newly discovered symbionts, a glimmer of hope emerged: with SST’s continuing to rise, corals may begin associating with more thermally tolerant symbionts. Indeed, evidence supporting this notion surfaced in 2006 when the abundance of Durusdinium, a genus renowned for its natural thermal resilience (think durable), increased within the tissues of corals subjected to repeated bleaching episodes. As a direct result of this switch, the thermal tolerance of these corals was predicted to have increased by an astonishing 1-1.5 °C likely helping them to endure these bleaching events.
Unfortunately, hosting naturally thermo-tolerant symbionts leaves corals caught between the devil and the deep blue sea. Long-term observations of coral-Durusdinium symbioses reveal a reduced rate of photosynthate translocation, slower growth, and smaller eggs as consequences. As marine heatwaves constitute a multi-stressor event, doubts began to arise over the ability of naturally thermo-tolerant symbionts to provide the energy necessary for corals to survive in a rapidly warming climate.
It all sounds quite disheartening. But what if I told you there was another way? A way where we could push beyond the limitations of coral-zooxanthellae symbiosis, boosting coral thermotolerance without the trade-offs to growth or longevity? These questions lie within the domain of experimental evolution – a powerful tool already used to boost the thermo-tolerance of many marine microalgal species.
Imagine for a moment you and nine of your close friends have stepped into a sauna. After five minutes, beads of sweat have begun to form on your skin as the air becomes thick with heat. Are you comfortable? Not really, but you’re not entirely uncomfortable either. 3 minutes later and it’s a different story. Sweat has trickled into your eyes, warm and stinging, the thumping of your pulse resonates in your ears like a relentless drumbeat. Hang in there and find your breath. Ninety seconds later and you can feel yourself adjusting to the heat. Cautiously, you open your eyes and find only six of your friends remain. Too hot to handle, 3 exited the sauna. You’ve survived the first round.
The reward for your perseverance? Well, just as you catch your breath, a scientist walks into the room, peers at you through the sauna’s glass door, and casually turns the temperature dial up by a single degree. Back to it, and eight minutes later only 5 of you remain. The dial gets bumped up another degree, 3 left. Another degree.
This relentless escalation continues, degree by agonizing degree, until a target temperature is achieved. For the lucky zooxanthellae under investigation in the van Oppen lab, that target was a sweltering 31 degrees – equivalent to a particularly stressful day on the reef. Yet, after a year of being subjected to this thermal selection process, those surviving, battle-hardened zooxanthellae were anything but stressed.
Much like the ancient Mesoamericans who selectively bred corn over millennia, choosing only the largest seeds to cultivate crops that would eventually dwarf their predecessors, the gradual temperature increases in this experiment ensured only the most heat-tolerant symbionts survived to the next stage. These heat-loving zooxanthellae weren’t just proficient at acclimatising either; throughout the course of the experiment their very genetic makeup underwent significant changes. Through deletions, insertions, rearrangements, and duplications, they evolved tangible thermal adaptations capable of being inherited by future generations. Dubbed Selected Strain (SS) these symbionts have since become the focus of intensive research for the past eight years.
As you may be thinking, developing thermo-tolerant zooxanthellae in the lab represents only one side of the equation. For these SS symbionts to provide any ecological benefit, those thermal adaptations accrued in the test tube must be conferred to the coral host. Otherwise, what’s the point?
To investigate this, all you need is a couple of litres of SS symbionts, some aposymbiotic coral recruits (apo, derived from Greek for “without”) and a world-class aquatic laboratory. Pale and desperate for symbionts, these hungry corals are ready to accept just about whatever is given to them and, as fortune would have it, you have just dumped ~ 75 million SS symbionts into their tank. Over the next few days, these foreign, lab grown symbionts will swim around care-free in their new environment before eventually being captured and ingested by the young corals.

Fig. 5
Diagram illustrating the chemical bleaching
process and reinoculation
Once nestled comfortably within the symbiosome, these symbionts promptly commence their primary function, photosynthesis and division and soon, every available space is packed tight with symbionts. Congratulations you have successfully established an SS-coral symbiotic relationship. Now let’s turn up the heat and put this new symbiotic bond to the test.
The findings so far are promising. Corals hosting SS symbionts exhibit remarkable resilience to thermal bleaching events, marked by reduced stress, lower mortality rates, and faster recovery rates. Notably, the presence of SS symbionts doesn’t hinder coral growth either, distinguishing them from those naturally thermotolerant symbionts discussed above.
So, let’s get a move on, right? Put these symbionts to work? Well, up to now, the positive impacts of SS symbiont-coral associations have been limited to the controlled confines of the laboratory. In this scenario, a handful of corals are plucked from the reef, transported to the lab, forced into an association with SS symbionts, and then redeployed back onto the reef. For a coral restoration method to truly make a difference however, it must operate on a grand scale and, as it so stands, the potential outcomes stemming from our current efforts represent a mere drop in the ocean. So, how do we upscale this tool? Specifically, how do we establish SS symbiont-coral associations on an ecologically relevant scale?
Here is where I propose a closer examination of “free-living” (FL) symbionts (i.e., symbionts living outside a coral host) and the concept of horizontal transmission.
FL symbionts are ubiquitous on coral reefs; they can be found floating in the water-column, buried within the sediment, nestled tightly in the faeces of corallivorous fish, or dwelling on the surfaces of macroalgae. Collectively this ragtag group of FL symbionts form what is referred to as the ‘symbiont reservoir’.

Fig. 6
Image showing symbiont reservoir
Approximately 85% of all coral species directly rely on this reservoir. These species are horizontal transmitters, that is, they must acquire their symbionts de novo from the external environment. As you can imagine, the physiology and diversity of symbionts within this reservoir is thus critically important; incompatible or thermally sensitive symbionts in a corals’ surrounding reservoir will not only impact its physiology, but its resilience and recovery from marine heatwaves.
Unfortunately, the outlook under current climate projections seems grim with natural populations of FL symbionts – and, by extension, the symbiont reservoir – unlikely to meet the demands of a warming planet. However, fortunately for us, and for corals, the reservoir’s composition is not predetermined; it is dynamic, and what’s more, it can be altered.
By enriching the symbiont reservoir with a substantial influx of engineered, FL SS symbionts, we can essentially stock the shelves with a novel coral superfood specifically designed to be utilised during periods of thermal stress. But let’s not get ahead of ourselves, how do we introduce SS symbionts into the reservoir and subsequently, into the polyps of the consumer in the first place?
Corals, though they may not look like it, function much like master accountants, meticulously balancing their books to ensure long-term sustainability of their enterprise. At the core of this balancing act lies a strict population management strategy enforced upon their symbiotic inhabitants, akin to an internal audit, whereby excess symbionts are identified and expelled into the water column. How a coral selects which symbionts to expel and in what quantity is influenced by numerous factors but generally equates to around 0.1% of the coral’s overall symbiont population per day. While this number might seem insignificant, considering that corals routinely host over 1 million cells per square centimetre and some colonies cover areas exceeding 30 square meters, the scale quickly becomes apparent.
It’s no wonder then that expelled symbionts represent the primary source of FL symbionts in the reservoir. However, beyond this, expelled symbionts represent a crucial window of opportunity for scientists. Building on this idea, let’s explore the exciting possibilities unlocked by the expulsion process.
As we know, a coral containing Durusdinium symbionts will expel Durusdinium symbionts. But what about a coral manipulated in the laboratory to contain SS symbionts? Will they expel SS symbionts? This is where things get interesting. If corals can indeed expel SS symbionts, all of a sudden, a pathway is created that would permit the injection of unimaginable numbers of SS symbionts into the reservoir.
The story doesn’t end here either. As these expelled SS symbionts journey through the reservoir, they become available for acquisition by surrounding corals on the reef. If a prospective coral likes the look of these shiny new symbionts and decides to incorporate them into their symbiosomes, it too will eventually begin expelling these symbionts. Can you see the pattern? Much like the spread of a contagious disease, the introduction of SS symbionts into the reservoir has the potential to spark a continuous cycle of infection and dissemination as these symbionts move from coral to coral, reef to reef. If such a perpetual exchange process could be established, scientists would theoretically be granted the ability to rapidly and with relatively minimal effort, enhance a reef’s resilience to environmental challenges on a grand and enduring scale.
Before we dive in, let’s hit pause for a moment and establish a few prerequisites this grand scheme must fulfill in order for it to have any chance of success. First and foremost, it’s crucial that corals hosting SS symbionts have the ability to expel these symbionts. Should we find that corals manage their population of SS symbionts through means other than expulsion, this idea is essentially dead in the water, and we might as well stop the story here.
If, however, we are lucky and corals do indeed expel SS symbionts, the next requirement pertains to the health of these symbionts. Stressed symbionts, those lacking cellular integrity, or perhaps most importantly, those unable to photosynthesise effectively, make very poor candidates in the already competitive symbiont reservoir. Therefore, it’s imperative that SS symbionts are not only expelled but also healthy and capable of photosynthesis
The final prerequisite, at least for now, hinges upon the expelled SS symbionts’ capacity to perform cellular division. If an SS symbiont can divide after expulsion, it paves the way for establishing a permanent, self-sustaining population of SS symbionts in the reservoir. A self-sustaining population would not only increase their concentration in the reservoir but also enable them to spread over greater distances.
These questions formed the basis of the first experiment of my PhD candidature. Taking a handful of corals possessing SS symbionts in their tissues, I meticulously collected and analysed their expelled symbiont population over 24-hours periods. What was the verdict? SS symbionts were expelled – check. They were healthy and photosynthesising – check. Dividing – check. A promising start. Now, the most important question: can they find home in another coral?
This brings us to the second experiment of my PhD affectionately titled The Raceway. Halfway between a bowling alley and a wave pool, The Raceway was a novel experimental system, engineered to determine whether expelled SS symbionts can be acquired in adult corals.
The beauty of The Raceway lies in its unique design: firstly, the twelve 40 cm long and 5 cm wide lanes are entirely self-contained and independent safeguarding against potential contamination. Secondly, the gentle flow of water within each lane is meticulously calibrated to mimic the natural rhythms of the reef. Lastly, with a unidirectional, non-recirculating current, we ensure that seawater entering The Raceway embarked on a one-way journey, idling past the corals before plunging down the drain with a gentle gurgle.

Fig. 7
Diagram showing raceway

Fig. 8
Diagram showing raceway
Now, onto the experiment. At the top of each lane, before the water inlet valve, we carefully placed one of the SS-harbouring corals painstakingly characterised earlier in Chapter 1, henceforth dubbed a Donor. At equal distances directly downstream we then positioned five Recipients; – corals of the same species, chemically bleached to remove their symbiont population.
The reasoning behind using bleached corals as Recipients as opposed to healthy ones is that bleached corals are starving corals, and as such will be more likely to ingest FL symbionts. As this is the first experiment of its kind investigating expelled SS symbiont uptake we decided to start with the lowest hanging fruit.
With the Donors and Recipients in place, the experiment begins. Immediately, morphologically viable, photosynthesising SS symbionts are expelled from the Donor to begin their short odyssey downstream on the current.
“It’s a dangerous business, Frodo, going out your door. You step onto the road, and if you don’t keep your feet, there’s no knowing where you might be swept off to.” (Bilbo Baggins, The Lord of the Rings: The Fellowship of the Ring)
Where indeed, for between the Donor and the plug at the end of The Raceway are five hungry, ghostly corals, their probing tentacles poised to seize anything that comes close. But will they be interested in our SS symbiont?
3-months later it was time to find out. Armed with nail-clippers seemingly designed for the sole purpose of extracting match-head sized pieces of coral, we sampled each of our Recipients for genetic analysis. After a month of nerve-wracking lab work, we had our answer. Two out of 45 Recipients. 4.4%. Our SS symbionts had made it in. We had successful horizontal transmission.
Although these numbers may not seem significant, this finding represents the first observation of laboratory-generated, heat-evolved symbionts being horizontally transmitted to adult corals. And while our story now ends, it represents only the beginning of this exciting journey into understanding and enhancing coral resilience through assisted evolution. With further research and interventions, we hope to provide corals with the breathing room they need to adapt and thrive in a warming environment.
About the Author

Bede Johnston is a PhD candidate in coral biology and restoration at the University of Melbourne, conducting research remotely at the Australian Institute of Marine Science (AIMS) in North Queensland. He holds a degree in Advanced Science from the University of Sydney and has previously contributed to studies on platelet clotting disorders in diabetes and genetic engineering techniques for Australian crops. His current research explores the biomolecular adaptations of experimentally evolved heat-tolerant symbionts and investigates methods for upscaling their application in coral reef restoration, with a focus on mitigating the impacts of climate change on coral ecosystems.